INO is trading $2.55, down 11.0%. The Symbol Summary is included below.

Provided by Livevol
Inovio Pharmaceuticals, Inc. is engaged in the development of a new generation of vaccines, called synthetic vaccines, focused on cancers and infectious diseases. The Company's SynCon technology enables the design of universal vaccines capable of providing cross-protection against existing or changing strains of pathogens, such as influenza and human immunodeficiency virus (HIV).
This one actually doesn't trade options and I almost never write about non option related companies, but there are a few things about this firm that make it worth a quick look:
1. The equity price is nearing the level of an option in and of itself (read: Merton Model).
2. This was the best performing bio-tech in 2013, up more than 400% last year.
3. I haven't seen this much interest in a bio-tech in social media in a long time.
4. It's down today.
5. I'm considering taking a position.
So, here we go with some info on INO. Let's start with The Charts Tab (two-years), below.
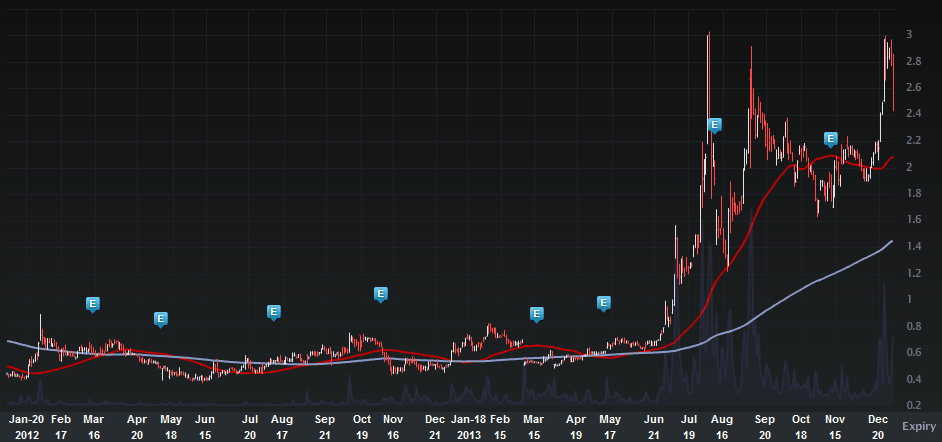
Provided by Livevol
So, your eyes aren't deceiving you, there are stock prices in the $0.40 range there. Anyway, here's the deal with INO, it is a fascinating firm in many respects. Two news articles kind of do the work for us:
The first article:
Source: TheStreet.com via Yahoo! Finance
Biotech Stock Mailbag: Inovio, Chelsea, Exelixis, Ariad, ImmunoCellular, written by Adam Feuerstein.
---
Inovio is conducting a randomized, placebo-controlled phase II study of its DNA vaccine VGX-3100, which enrolled 148 women with cervical dysplasia, also known as cervical intraepithelial neoplasia (CIN). Results are expected to be announced mid year.
All the women enrolled in the study are confirmed positive for the human papillomavirus (HPV) serotypes 16 and 18, [] which carry a moderate to higher risk for progressing to cancer.
CIN, by itself, is not cancer. These are pre-cancerous cells, which if untreated, might turn into cervical cancer. []
VGX-3100 is a therapeutic DNA vaccine delivered using Inovio's electroporation device. It's designed to produce a T-cell response that identifies and kills cells that have been transformed by HPV into precancerous lesions.
[]
The study's primary endpoint is a comparison of the number of patients with regression of CIN to grade 1 or less at nine months. The secondary efficacy endpoint of the study is the number of patients with clearance of HPV 16 or 18 and regression CIN to grade 1.
I'm an Inovio skeptic, with good reason. The technology upon which the company's vaccine candidates are based is more than 30 years old and has never proven to be effective in clinical trials. Inovio's management team is exceptionally promotional and 85% of the company's stock is held by retail investors, according to S&P CapitalIQ. [Promotional efforts plus speculation plus a raging biotech bull market explain much of Inovio's stellar performance in 2013.]
In September, Inovio and Roche (ROG) formed a partnership to develop vaccines against prostate cancer and hepatitis B. But the products involved in this deal are both still in preclinical stages of development and Inovio only received a $10 million upfront payment -- which amounts to a penny-sized financial commitment for a company the size of Roche.
---
That was semi-bearish. Here's another article, a bit more bullish (yellow highlighting was added by me):
Why You Should Watch Amgen and Inovio Today, written by George Budwell.
---
So what's driving the share price appreciation? While Inovio has a number of promising pre-clinical and early stage vaccine candidates, my take is that investors are squarely focused on the company's lead clinical candidate VGX-3100. VGX-3100 is a synthetic DNA vaccine being tested in a mid-stage trial as a possible treatment for cervical dysplasia. Inovio is expected to release topline results from the study by mid-2014.
Why is this vaccine worth keeping tabs on? Simply put, VGX-3100's clinical results will either validate the company's entire synthetic vaccine platform, or possibly sink it. So while VGX-3100 could be a blockbuster in its own right due to the size of the potential market, this trial has much broader implications for Inovio as a whole. If the trial is successful, it validates Roche's recent licensing deal with Inovio that could generate up to $422 million in milestone payments, and it would significantly de-risk the company's other clinical candidates for HIV, Influenza, and other cancer types.
My take is that the company's market cap of roughly $600 million is factoring in the significant risk of the upcoming data readout. Other cutting edge vaccine-makers like Novavax, for example, are commanding a much higher premium right now. In short, Inovio still looks cheap based on prevailing sector trends despite its monstrous rise in 2013, but this bargain isn't risk-free. Foolish investors thus might want to keep tabs on this one going forward.
---
My take (not that it matters at all):
Bio-techs at this stage really are pure gambles. There's a general line I take which is simply that I hope they're all successful at eradicating disease, but hope is worth 1 cent less than a penny in the stock market. As the price dips (if it continues) it makes the equity price an option in and of itself. Bet carefully...but watch this one, it' going to make news this year, one way or the other.
This is trade analysis, not a recommendation.
Follow @OphirGottlieb
Tweet
Legal Stuff:
Options involve risk. Prior to buying or selling an option, an investor must receive a copy of Characteristics and Risks of Standardized Options. Investors need a broker to trade options, and must meet suitability requirements.
The information contained on this site is provided for general informational purposes, as a convenience to the readers. The materials are not a substitute for obtaining professional advice from a qualified person, firm or corporation. Consult the appropriate professional advisor for more complete and current information. I am not engaged in rendering any legal or professional services by placing these general informational materials on this website.
I specifically disclaim any liability, whether based in contract, tort, strict liability or otherwise, for any direct, indirect, incidental, consequential, or special damages arising out of or in any way connected with access to or use of the site, even if I have been advised of the possibility of such damages, including liability in connection with mistakes or omissions in, or delays in transmission of, information to or from the user, interruptions in telecommunications connections to the site or viruses.
I make no representations or warranties about the accuracy or completeness of the information contained on this website. Any links provided to other server sites are offered as a matter of convenience and in no way are meant to imply that I endorse, sponsor, promote or am affiliated with the owners of or participants in those sites, or endorse any information contained on those sites, unless expressly stated.



No comments:
Post a Comment